Ultra-sensitive
As low as 1 ppm of limit of detection (LOD95)* delivering 100-1,000x greater sensitivity than existing MRD approaches
*At simulated >10,000 mutations, 100x read-depth
Panel free
Go beyond personalized panels with our whole genome baseline x whole genome monitoring, capturing rare variants in a complex tumor environment
Clinically validated assay
Rely on enhanced accuracy and reliability from a clinically validated assay performed by a CAP/CLIA laboratory, ready to use for both clinical and research settings
More data & insights
Get highly curated whole genome cancer profiling data along with MRD assay report – deeper insights for deeper exploration
Whole genome x whole genome innovation
MRDVision provides an exclusively whole genome approach, delivering a simplified workflow and ultra-precise monitoring.
Unlike existing MRD assays that rely on creating panels with a limited number of genes for each patient, MRDVision preserves personalized whole genome insights from whole genome cancer profiling data (our CancerVision™ platform) continuously scanning the entire genome to reduce the likelihood of missing tumor signals without being limited to pre-selected targets.
No panel for each patient, which is costly and time-consuming
Greater confidence in capturing rare variants in a complex environment
How MRDVision works
Ultra-sensitive MRD detection
Merging two advanced technologies — CancerVision, a solid tumor cancer whole genome platform by Inocras, and Ultima Genomics’ ppmSeq™, MRDVision has shown ultra-high sensitivity.
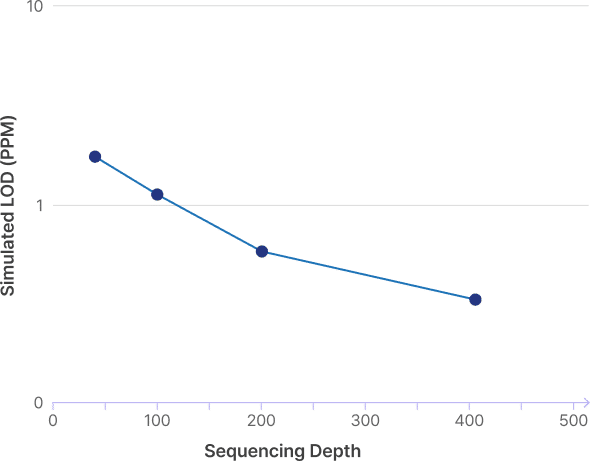
MRDVision limit of detection (LOD95)
MRDVision demonstrated a simulated 95% of limit of detection (LOD95) as low as 1 ppm at >10,000 mutations and 100x read-depth
Industry-leading performance, demonstrated in head-to-head comparison
In its clinical validation study using cfDNA samples from pre- and post-surgical ovarian and lung cancer patients, MRDVision demonstrated high concordance with an orthogonal test and detected tumor-derived signals even at low tumor fractions.
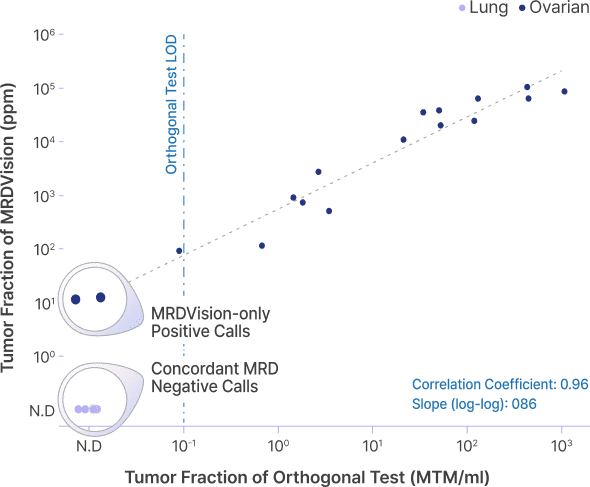
MRDVision clinical validation with head-to-head comparison
- MRDVision has shown strong quantitative concordance with a competitor assay
- In 6% of cases, MRDVision detected tumor-derived signals that were not identified by the competitor assay
Not all MRD tests are created equal – experience the MRDVision difference
| Key features | MRDVision | Widely adopted MRD products |
|---|---|---|
| Product concept | Tumor-informed | Tumor-informed |
| Genome coverage | Baseline: Whole genome based (CancerVision) ctDNA: WGS | Baseline: Whole exome or whole genome based ctDNA: Personalized or fixed panel with limited number of variants |
| Limit of detection | 0.0001% (1 ppm) LOD95: as low as 1 ppm at simulated >10,000 mutations, 90x read-depth | Mostly 0.01% - 0.001% market-wide |
| Deliverable | WGS ctDNA monitoring report + Whole genome cancer profiling data (CancerVision) | ctDNA monitoring report |
| Turnaround Time | First order: 2-4 weeks Follow-ups: 7-14 days | First order: 4-5 weeks Follow-ups: 7-14 days |
| Price | More economical due to simple workflow innovation | Higher price point with panel creation steps |
Use MRDVision insights to accelerate innovation
For biopharma
Accelerate your clinical development with MRDVision
Patient stratification
Stratify and enrich patient subgroups who are most likely to benefit from novel therapies, driving more precise study design
Treatment determination
Use MRD status to monitor drug response and determine treatment incl. dosage escalation or de-escalation
Surrogate end-point
Accelerate a clinical trial timeline using MRD as a surrogate marker to track early treatment response
One supplier for tumor profiling and MRD
Consolidate tumor profiling and MRD testing all into one supplier with whole genome insights
For cancer researchers
Unlock the full potential of your cancer research
Novel biomarker discovery
Discover and validate novel, predictive biomarkers, using comprehensive genomic data from MRD test
Therapeutic impact study
Exploring the therapeutic impact at a molecular level, detect resistance mechanisms, and monitor tumor evolution over time
Advanced liquid biopsy research
Advance liquid biopsy research with ctDNA WGS to pioneer new approaches in non-invasive diagnostics and track genetic alterations with high sensitivity
Impact with scale
Scale your research with our competitively priced MRD assay – without compromising on precision or scope
Intended use: To track ctDNA using a tumor-specific genomic fingerprint to detect MRD in patients with solid tumors and lymphomas.
Sample requirements and turnaround time (TAT)
First test: Baseline CancerVision + MRDVision – within 2 to 4 weeks
Tumor tissue:
- FFPE tissue: ≥10 slides in 5 micron thickness with an H&E stained slide for reference, shipped ambient
- Fresh frozen: 25mg of cryopreserved tissue, shipped frozen on dry ice
Matched normal and cfDNA
- Blood: 2 Streck tubes of peripheral blood (10mL each), shipped ambient or with cold packs (must be received within 7 days of collection)
From second test: MRDVision – within 7-14 days
- 2 Streck tubes of peripheral blood (10mL each), shipped ambient or with cold packs