Comprehensive cancer profiling to guide your patients’ most important health decisions
Get the data you need to support your best clinical decision making.

By the numbers
421+
Lorem ipsum dolor sit amet
Korem ipsum dolor sit amet, consectetur adipiscing elit. Nunc vulputate libero et velit interdum, ac aliquet odio mattis.
421+
Lorem ipsum dolor sit amet
Korem ipsum dolor sit amet, consectetur adipiscing elit. Nunc vulputate libero et velit interdum, ac aliquet odio mattis.
421+
Lorem ipsum dolor sit amet
Korem ipsum dolor sit amet, consectetur adipiscing elit. Nunc vulputate libero et velit interdum, ac aliquet odio mattis.
421+
Lorem ipsum dolor sit amet
Korem ipsum dolor sit amet, consectetur adipiscing elit. Nunc vulputate libero et velit interdum, ac aliquet odio mattis.
CancerVision
CancerVision provides broader insight than many conventional genetic tests and reduces the likelihood of false positives by ruling out benign variants in the tumor sample.
Who is CancerVision for?
CancerVision is a whole genome sequencing test for patients with solid tumors that may have been recently diagnosed, are recurring or metastatic, or aren’t responding to treatment. It is also for patients who want to find more answers about the genetic drivers of their cancer. We offer you and your patients whole genome coverage, actionable findings, clinical trial matching and expert support to provide the best care possible.
We relieve your diagnostic burden so you can spend more time caring for your patients
Our goal is to be a resource for you beyond top-tier genetic testing. We’re here to provide and translate complex data and findings to turn them into actionable insights to support your patients.
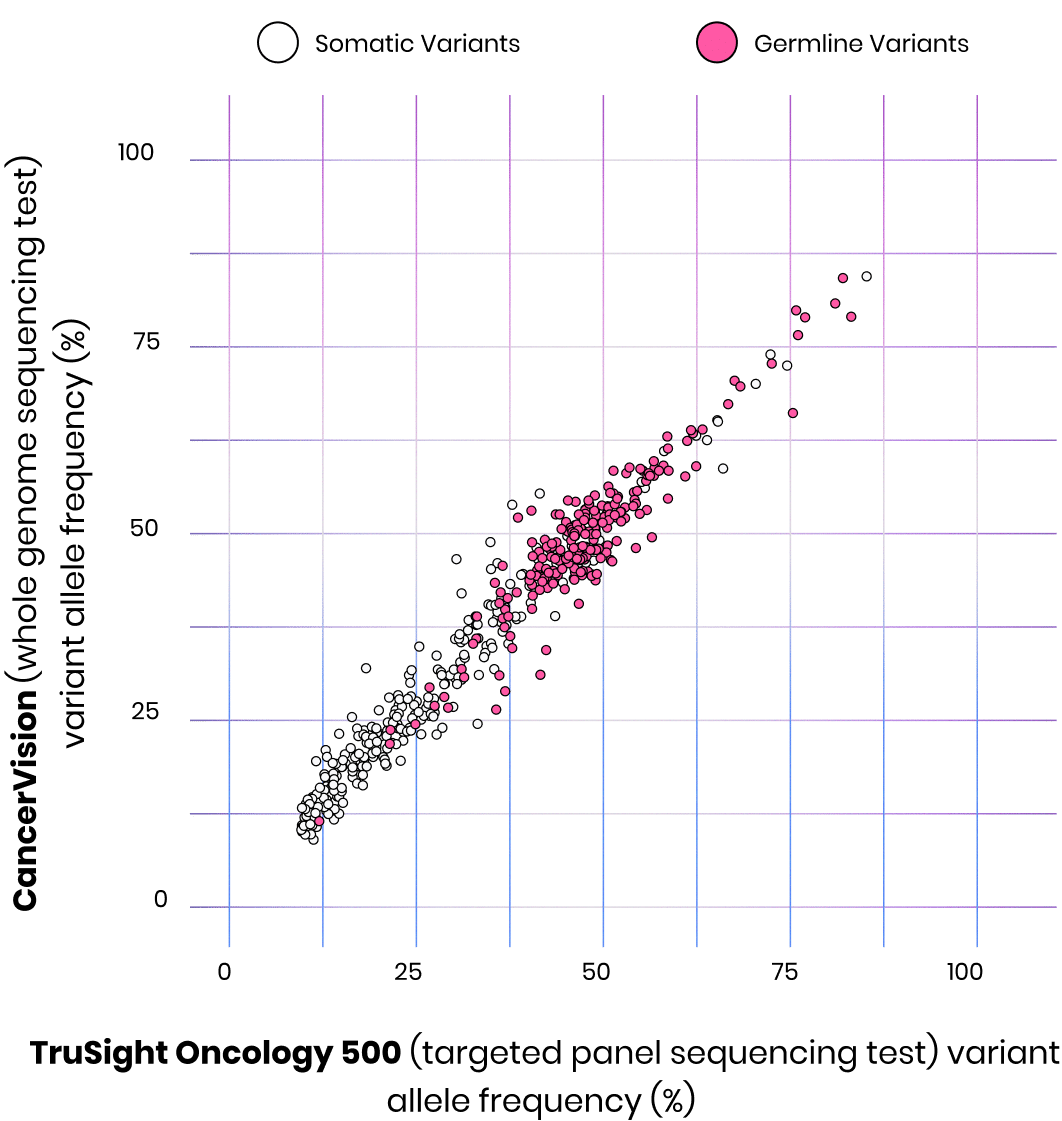
We capture more than what NGS captures
CancerVision has been clinically validated and detected 100% of the variants1 identified by the clinically validated TruSight Oncology 500 (Illumina) NGS target oncology panel test. Additionally, CancerVision distinguished between germline and somatic variants. Understanding the origin of the mutation is critical in choosing the most effective treatment options.
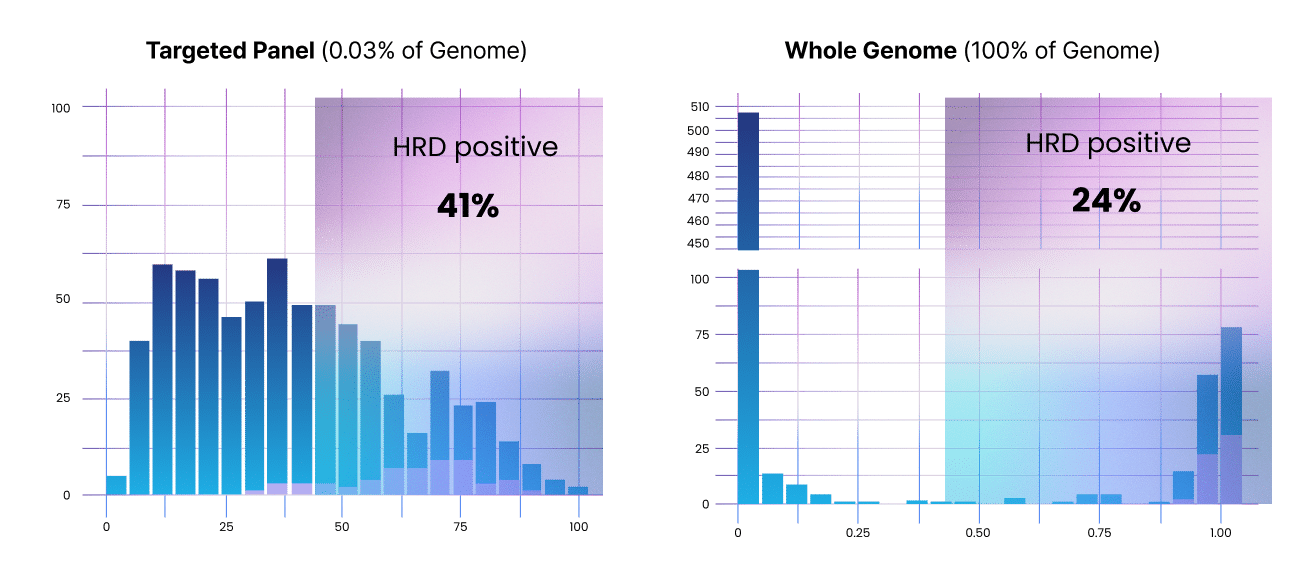
We provide highly sensitive genome-wide signatures
Whole genome sequencing finds more complex changes within the genome. For example, CancerVision is able to detect accurate genomic pattern-based markers and provides highly accurate HRD detection – without adding any other tests (688 cancer patient cases).
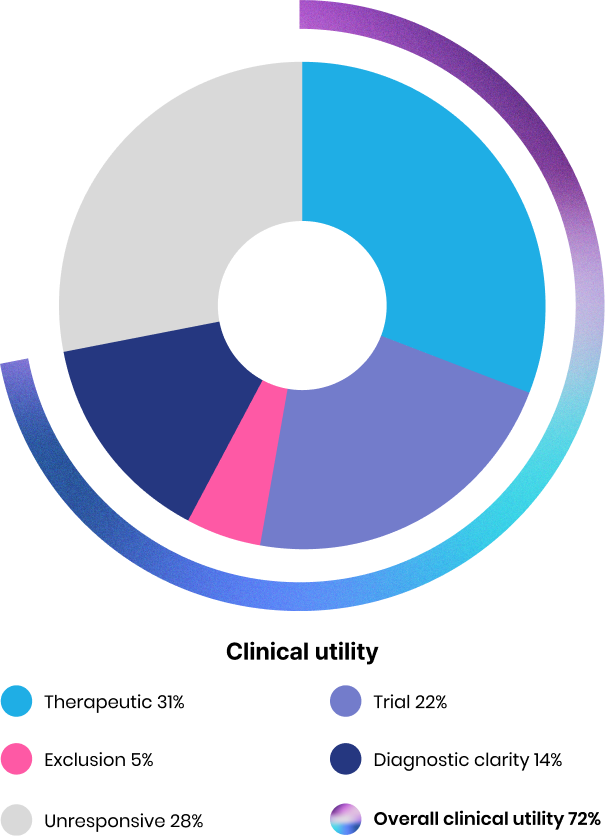
Actionable insights
Our proprietary high-performing bioinformatics algorithm thoroughly analyzes a patient’s genome data and highlights the findings highly relevant to clinical utility. In one study, more than 70%2 of our WGS reports yielded clinically relevant insights, including actionability for therapeutic options and clinical clarity.
How to order a test
Ordering a test is quick and easy in our provider order portal.
Start an online order by filling out the test requisition form. We will send our kits to your office.
Use our supplied sample collection kit and instructions to collect samples for testing.
Ship the samples directly to us.
Receive the test results via email and fax. You can also access to all test results you ordered in our online portal.
Test requirements
Specimen
10 FFPE from curls or slides, with an H&E stained slide for reference
Paired normal
2 Streck tubes of peripheral blood (10mL each) or one buccal swab in fixative solution
Turnaround time
2 weeks from receipt of patient samples at our lab
Provider resources
Frequently asked questions
If you don’t see your question answered here, please contact us at inquiry@inocras.com.
How do I order CancerVision?
To order a test, please sign up and complete the online test requisition form. Our sales representatives will follow up with you shortly.
How are the tests paid for?
Our tests are not currently covered by insurance. Upon ordering, your patient will receive an invoice for which payment will be required directly from the patient’s out-of-pocket expenses.
How will I receive the results?
You can access Inocras test reports as soon as they are ready via our digital provider order portal. You can also receive access to results via secure email or request to have results faxed to you.
What if I have questions about my patient’s results?
You’ll have access to our team of in-house medical experts, researchers, and genomic scientists who can explain the results to you and answer any questions. If you have questions about a patient’s results, you can email us at inquiry@inocras.com for support. One of our experts will respond to your questions or set up time to speak with you.

