Genetic Testing Uncovers Li-Fraumeni Syndrome in a Breast Cancer Patient

Context and Challenges
A 40-year-old female presented with a palpable mass in her right breast, initially detected during a routine health check-up. Subsequent clinical examination, imaging, and biopsy confirmed the diagnosis of breast carcinoma. Given her medical history, which includes a previous sarcoma diagnosis in her twenties, comprehensive genetic testing was warranted to refine the diagnosis and guide personalized management strategies.
Solution and Outcomes
The treating physician ordered CancerVision’s whole-genome sequencing (WGS) powered comprehensive genomic testing. CancerVision provided actionable insights by analyzing both the patient’s tumor (somatic) and germline (heritable) genomes.
The CancerVision analysis revealed a structural variation in the TP53 gene, a critical tumor suppressor (Figure 1). Structural variants (SVs) are large-scale alterations in the genome involving rearrangements of DNA segments, including deletions, duplications, insertions, inversions, or translocations. These changes can affect thousands to millions of nucleotides, significantly impacting gene function and contributing to various genetic disorders and cancers.
Through the power of WGS, CancerVision can effectively detect structural variants by providing a comprehensive view of the genome. Unlike targeted sequencing methods, such as Targeted Panel Sequencing (TPS) or Whole Exome Sequencing (WES), which focus on specific genes or regions, WGS captures the genomic landscape. This allows WGS to identify complex structural variations that may be missed by tests limited to smaller, targeted portions of the genome.
The identification of the TP53 structural variant, combined with the patient’s medical history, led to a definitive diagnosis of Li-Fraumeni Syndrome (LFS). LFS is a rare, inherited disorder characterized by a significantly increased risk of developing multiple types of cancer. It is caused by pathogenic mutations in the TP53 gene, which may include point mutations (single nucleotide changes) or structural variations (large-scale alterations). Mutations in the TP53 gene disrupt its role in regulating cell growth and preventing tumor formation, increasing the risk of cancers such as breast cancer, sarcomas, brain tumors, leukemia, and adrenal gland carcinomas. LFS follows an autosomal dominant inheritance pattern, meaning a mutation in one copy of the TP53 gene is sufficient to raise cancer risk. Individuals diagnosed with LFS may require intensive surveillance and screening programs to detect cancers early and manage risks effectively. Additionally, it is important for family members of individuals with LFS to consider genetic testing to determine if they have inherited the TP53 mutation. Screening at-risk relatives allows for an accurate assessment of their cancer risk and enables the implementation of early surveillance and preventive measures when necessary (1).
Conclusion
The identification of LFS in this patient highlights the importance of comprehensive genetic testing, including both somatic and germline analysis, in individuals with early-onset or recurrent cancers and a strong family history. Evaluating both types of mutations offers a robust genetic profile, to guiding personalized treatment and risk management for the patient and their family. Regular screenings and genetic counseling based on these findings may improve outcomes through early detection and prevention.
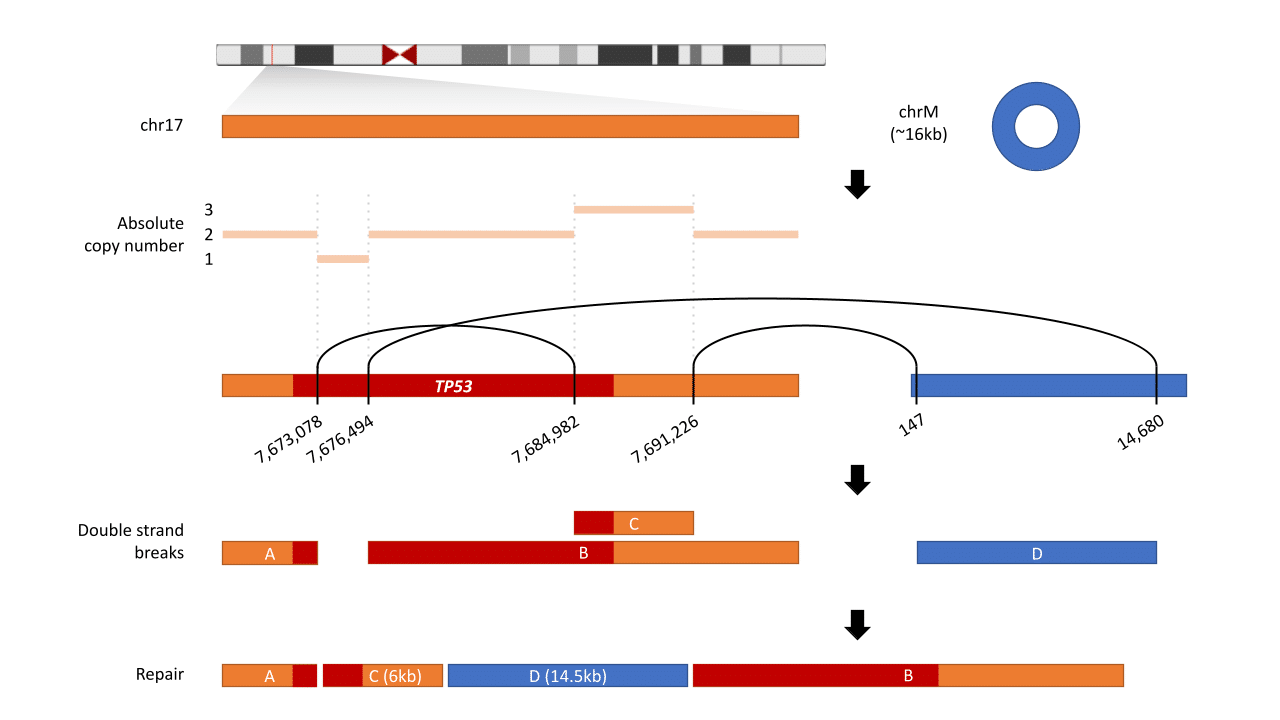
Figure 1: TP53 Gene Structural Variation The diagram depicts a structural variation in the TP53 gene on chromosome 17. Breakpoints are identified at positions 7,617,078-7,617,894 and 7,984,902-7,991,226, leading to double-strand breaks (DSBs). These breaks result in a rearrangement where segment D (14.5kb) from chrM integrates between segments C (6kb) and B. The figure highlights how WGS can detect these large-scale genomic alterations that may be missed by targeted sequencing methods.